The Next Chapter of Elevated Temperature Landfill Research
Dr. Mort Barlaz with the North Carolina State University (NCSU) went beyond the conceptual modeling of heat production from aluminum corrosion to simulating landfill relevant conditions in the laboratory. Dr. Barlaz discusses the complexity of simulating landfill conditions in the lab while being able to capture important information to be able to use gas volumes, hydrogen (H2) gas formation, and the reaction time to estimate the extent and rate of aluminum corrosion.
February 17, 2022

A common theme among attendees at GWMS was the excitement to see our friends and colleagues and talk about waste in person after two years of online meetings. In the “Elevated Temperature Landfills” session the common theme was a little different. The attendees were excited to come together to see the clear value of solid waste research after multiple years of elevated temperature landfill (ETLF) studies. These studies were primary funded by the Environmental Research & Education Foundation with industry support. These studies provided industry with the information necessary to act quickly to address this issue and continue to be proactive in the management of ETLFs. This session was moderated by Michael Beaudoin with Republic Services. The overarching theme of the research presented revolved around understanding how ash contributes to the generation of heat in a landfill.

Dr. Debra Reinhart with the University of Central Florida kicked of the session with a study that aimed to understand how the characteristics of coal combustion ash (CCA) and municipal solid waste incinerator (MSWI) ash contributed to the generation of heat. Previous research on field data from ETLFs and non-ETLFs showed that when ash was disposed with MSW there was a noticeable increase in the max temperature observed in the field relative to landfills without ash.
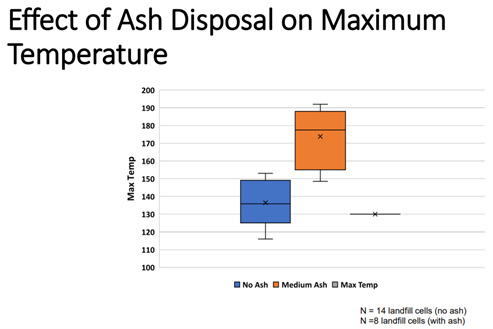
She went on to explain the differences in characteristics of CCA and MSWI ash and how this will change depending on the source of the ash or how it was generated. Using modeling approaches CCA may be expected to generate up to 15% more heat when compared to MSWI ash due to the differences in the composition. This study also highlighted how the relative heat potential of CCA was approximately six times greater when compared to the anaerobic degradation of simple organics.

Dr. Mort Barlaz with the North Carolina State University (NCSU) went beyond the conceptual modeling of heat production from aluminum corrosion to simulating landfill relevant conditions in the laboratory. Dr. Barlaz discusses the complexity of simulating landfill conditions in the lab while being able to capture important information to be able to use gas volumes, hydrogen (H2) gas formation, and the reaction time to estimate the extent and rate of aluminum corrosion. Aluminum corrosion was sensitive to the concentration of aluminum. This means that if a higher amount of aluminum is present then more heat could be produced. Leachate humic acid was also found to affect aluminum corrosion. Dr. Barlaz explained that the source of this material produces different effects on corrosion and that field leachate had higher rates. Since aluminum corrosion does occur within the first two to three days in the laboratory studies, it would be expected that a relatively large amount of heat can be produced over a short timeframe.
Another important pathway for heat generation in a landfill is ash hydration and carbonation. The hydration reaction occurs when water reacts with calcium oxide to produce calcium hydroxide. When calcium hydroxide goes on to react with carbon dioxide it undergoes carbonation. These reactions have been shown to produce heat in landfills from earlier research. Asmita Narod with NCSU is studying these reactions in ash under landfill relevant conditions by developing a reactor that will allow her to capture the heat released. Asmita’s studies have shown that 100% of the theoretical heat from hydration was able to be recovered in the reactors. The recovery of heat released from gaseous carbonation was 92-110%.
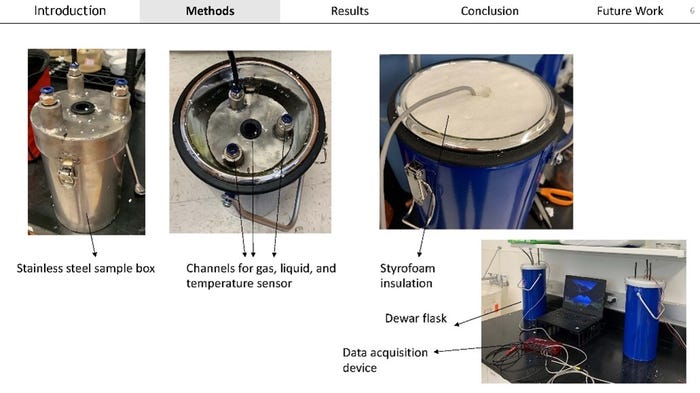
These presentations highlighted important information that is needed by the solid waste industry to know what to look for in incoming waste and how to adjust waste acceptance criteria.
About the Author
You May Also Like


