The Science of PFAS: Finding Strength in the Single Bond
May 13, 2021

To date, the large variation in the properties of PFAS compounds make it difficult to have a concrete understanding of exactly how they will behave coupled with the difficulty of being able to track and understand the over 4,600 PFAS compounds currently registered. If there is anything that can be learned from a quick search of PFAS it is that the carbon-fluorine (C-F) bond is one of the main properties that we can thank for the conveniences that these compounds offer, but also for the headaches that we experience when trying to figure out how to manage them. What is unique about the C-F bond and why is it different from any other chemical bond?
One of the main attributes of the C-F bond and also one of the main reasons for the love-hate relationship we have with PFAS, is the bond strength. In general, the bond strength dictates how easy it may or may not be for that bond to be broken. As the saying goes, there is strength in numbers and a similar statement can be made about the number of bonds in chemistry. As you increase the number of bonds from single, to double, to triple the strength of the bond increases. One way to think about this concept is attaching one rubber band to two balls and seeing how easy it is to pull apart those balls. What if you added another rubber band or even two to simulate the triple bond? What do you think happens? There is more resistance making it more difficult to pull apart relative to the balls attached with one rubber band.
Without increasing the number of bonds, chemists are able to use the various properties of elements to manipulate the strength of single bond. Organic chemists consider the C-F bond to the strongest single bond possible in organic chemistry. Therefore, the bond strength is the primary reason PFAS is known to be persistent in the environment, bioaccumulates, does not degrade in conventional treatment processes for both wastewater and drinking water, and is thermally stable. These properties are the reason PFAS is considered the “forever chemical.” Subsequent pieces will breakdown the science on how the number of carbons and functional groups also contribute to the complex nature of PFAS and how this impacts the management of these compounds.
What is it about the addition of fluorine that makes the bond so strong?
The reason for the strength of this bond is the electronegativity of fluorine and its relative attraction to carbon. Chemists use electronegativity as the measure of the ability for an atom (fluorine) to pull electrons away from another atom (carbon). This behavior is similar to a magnetic force pulling the electrons away from the carbon and towards fluorine causing a stronger bond and fluorine not wanting to give up those electrons. More specifically fluorine has a partial negative charge and carbon has a partial positive charge as shown below and as expected opposites will attract.
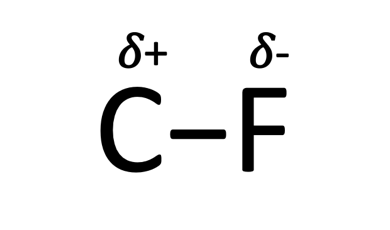
Looking further at a typical C-H bond relative to a C-F bond, we see that there is a 17% increase in the energy required to break the single bond when you replace H with F. Comparing the H-F and C-F bond to other common single bonds you see that the bond strength for C-F is still at the upper end relative to the other examples shown below.
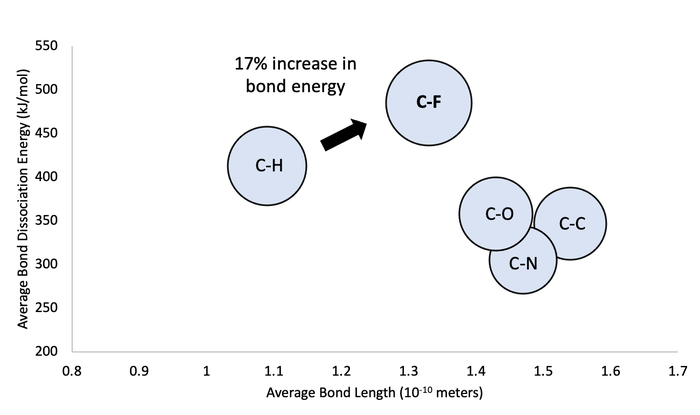
The interaction between C-F has created both unique properties as well as challenges in the management of PFAS compounds. Unlocking ways to break the bond barrier will be key advancements needed towards finding affective ways to manage these compounds in the environment.
About the Author
You May Also Like


