Setting the Stage for PFAS Research at GWMS
GWMS began with a knowledge packed session on per- and polyfluoroalkyl substances (PFAS) which was a topic that attendees were most looking forward to this year. The first PFAS session was on “PFAS Characterization” and was moderated by Joe Benco with Republic Services. This session focused on introducing PFAS issues and research more broadly and included topics that will be discussed in more detail in subsequent sessions.
February 16, 2022

GWMS began with a knowledge packed session on per- and polyfluoroalkyl substances (PFAS) which was a topic that attendees were most looking forward to this year. The first PFAS session was on “PFAS Characterization” and was moderated by Joe Benco with Republic Services. This session focused on introducing PFAS issues and research more broadly and included topics that will be discussed in more detail in subsequent sessions.
It is well known that the main reason why PFAS are a complex challenge for the industry is the strength of the carbon fluorine bond in addition to the widespread use of products containing these compounds. Dr. Stephen Zemba with Sanborn Head & Associates highlights that in addition to the strong bond between carbon and fluorine these compounds also have a water-loving head and a backbone that is both water and fat repelling (image below). Due to these characteristics PFAS tends to be found between the air and water interfaces.
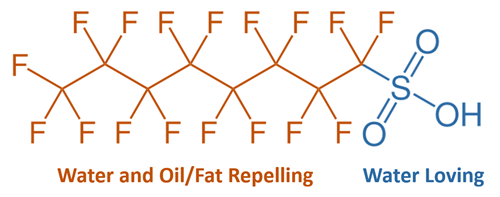
Dr. Zemba goes on to explain how these properties are attributed to the relative persistence in our bodies and the focus on research to understand the suspected health impacts. As legacy compounds have been voluntarily phased out and there is now a continued shift to shorter-chain compounds, it has been demonstrated that we are seeing consistent decreases in the blood serum levels overtime for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). The concerns over potential health impacts have driven regulators to consider initiatives to address the management of PFAS. The most noticeable initiatives that would affect the solid waste industry includes a hazardous substance declaration under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), ambient water quality criteria under the Clear Water Act, effluent limitations under the National Pollutant Discharge Elimination System (NPDES), and maximum contaminant levels (MCLs) under the Safe Drinking Water Act.
It is important to understand the mass flux of waste materials that will end up at a landfill and what the potential release of PFAS is to leachate, landfill gas, and stormwater. This information will inform how the pending regulatory efforts could affect the solid waste industry and provide information necessary for the industry to get ahead of these initiatives. Preliminary field studies by Dr. Zemba that calculated the flux of PFAS into a landfill versus PFAS in leachate have shown that landfills might act as a sink for PFAS compounds but to what extent is still unknown. Research being conducted by Mojtaba Sardarmehni at the North Carolina State University (NCSU) is working towards developing a conceptional model to estimate PFAS releases attributable to a wider range of materials containing PFAS and understand the cycling of these compounds between multiple management strategies.
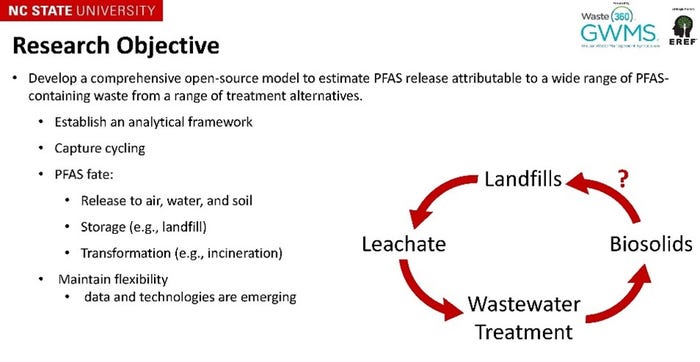
Terry Johnson with Waste Management expanded on the dynamic relationship of PFAS into a landfill and out with results from a long-term study with three landfills in the Midwest. The aim of this study was to provide insight into the trends of 13 compounds overtime. Results showed that with the voluntary phase out of PFOA and PFOS there was a downward trend in the concentration of these two compounds in leachate. This trend was similar for other long-chain compounds. On the other hand, the trends for the short-chain compounds were more variable but in general showed an increasing trend. This trend is supported by research that has demonstrated how various processes that occur within landfills can contribute to the shift in long-chain to short-chain compounds in leachate as well as the shift to shorter-chain compounds in products. Terry noted that perfluorohexanoic acid (PFHxA), a degradation product from stain- and grease-proof coatings on food packaging, couches, and carpets, had the most abrupt increasing trend. Focusing on upstream sources can help minimize exposure in the household but also the influx of PFAS to landfills.

Although the prevalence of PFAS in leachate is well documented, data on the presence of PFAS in landfill gas is lagging due to the lack of a validated methodology to collect and characterize PFAS in landfills gas. Dr. Florentino De la Cruz at NCSU is working diligently to develop and validate a methodology suitable to begin to characterize PFAS in landfill gas. Preliminary results have shown that landfill gas is dominated by fluorotelomer alcohols (FTOH). FtOH are volatile precursors of perfluorinated carboxylic acids (PFCA), which means they are more likely to move from the liquid to gas phase. Dr. De La Cruz goes on to discuss how this information can be used to determine the mass of PFAS in landfill gas and the potential mass released as emissions.
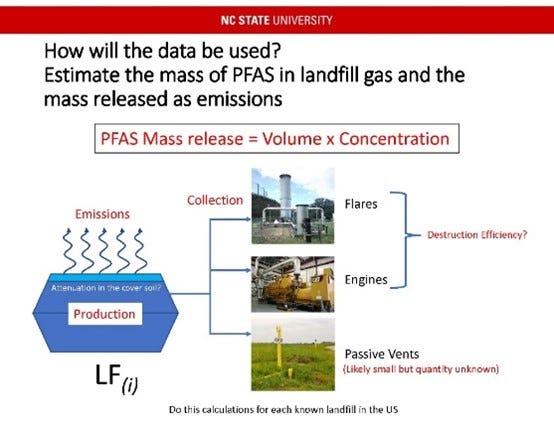
These presentations highlighted important advances in PFAS research that will work towards closing gaps in understanding the flux of PFAS into and out of a landfill through liquid and gaseous emissions.
About the Author
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

