The Role Of Battery Recycling In The Circular Economy: Part 1 - Key Technologies

Editor's Note: The Role Of Battery Recycling In The Circular Economy is a three-part series. Part 1 focuses on Key Technologies. Part 2 focuses on the Battery Supply Chain, Logistics and Profitability. Part 3 focuses on Challenges and the Role of Policy.
Only a few major battery innovations (Lead Acid by Plante, Nickel Cadmium by Jungner, Lithium-ion by Goodenough/Sony) have reached significant market penetration since the 1800s. As of 2018, over 90% of large‐scale battery storage power capacity in the US was provided by batteries based on Lithium‐ion (Li-ion) chemistries [1]. The demand for Li-ion batteries for consumer electronics and electric vehicles (EVs) is projected to grow about tenfold until the next decade. By 2025, the global revenue from Li-ion batteries is expected to reach $71 billion USD [2]. The volume of retired batteries follows an S-like curve, with less end-of-life Li-ion batteries today, but an estimated 315 GWh (1,619,000 tons) available for recycling by 2030 (assuming a lifetime of 10 years) [3], a volume roughly equivalent to current annual battery production [4].
Recycling Li-ion batteries is critical to address safety, environmental, and supply considerations. Retired batteries pose a fire hazard due to volatile components such as the electrolyte, particularly dangerous given the possibility of HF formation [5]. Additionally, an EV battery can be responsible for up to a third of the vehicle’s life-cycle emissions from cradle to grave [6]. Mining of raw materials for Li-ion batteries can be environmentally costly, as the process consumes excessive amounts of water, uses strong acids, and can contaminate underground stores of fresh water. Finally, there are varying predictions of critical supply shortages such as lithium, nickel, cobalt, and copper to meet EV demand. Recovering and recycling allows for more independence from geological mining and potentially a reduced cost of raw materials.
Many companies see the opportunity to turn the Li-ion battery waste problem into profit given to the increasing prices for Li-ion battery raw materials such as lithium, nickel, and cobalt. Currently, the Chinese market is well advanced in recycling (for example, Ganfeng has a capacity of 100,000 tons/year), followed by the European market (30,000-40,000 tons/year which includes Umicore and Glencore). The US needs to catch up in battery supply, refining, and recycling in order to be competitive. This article focuses on companies and organizations and how they fit into the Li-ion battery recycling ecosystem.
Key Technologies in Recycling
Li-ion batteries consist of a cathode, anode, electrolyte, separator, current collector foils, and packaging. Today’s Li-ion battery recycling companies primarily rely on some combination of two well-established processes, pyrometallurgy and hydrometallurgy. Direct recycling is a research-stage approach promising a shorter recycling loop at lower cost (see Figure 1). Electro-extraction (not shown) is in the early stages of deployment, focused on modularity and reduced costs and emissions by providing upgraded feedstock for the final stages of hydrometallurgy.
Pyrometallurgy, or smelting, is the process of melting battery packs or the shredded and separated cathode materials and reacting the molten metal oxides with carbon, which acts as a reducing agent to decompose the ore into metal, slag and carbon dioxide. Smelting has been used for centuries to refine ores into metals. In the context of Li-ion battery recycling, it is used today to recover elements such as copper, nickel, and cobalt. The benefits of smelting are that it is well-tested and simple, eliminating the need to shred or separate the Li-ion battery components. However, the process is very energy-intensive and materials recovery rates are significantly lower than competing processes. In particular, smelting is not suitable for the recovery of elements such as aluminum and lithium or the plastic packaging.
Further, the treatment of toxic air emissions (such as fluorine, phosphorus, sulfur, and particulates containing heavy metals) during smelting is costly. Pyrometallurgy is the predominant recycling technology used in China and Europe. In the US, Redwood Materials first collects batteries from a variety of partners. Rather than relying on fossil fuels for smelting, Redwood uses residual energy in the batteries to produce an alloy [8]. Afterwards, Redwood employs hydrometallurgical methods to reach recovery rates of 95-98% for nickel and cobalt (80% for lithium) [9]. This allows them to reach the quality necessary to sell their output raw materials to battery manufacturers.
“Today the EU [using pyrometallurgy] can achieve a Recycling Efficiency Rate (RER) of 50% (targeting 65-75%)...compare this to the Functional Material Recovery Rate (FMRR), which can be optimized economically but if you volatilize other components, like graphite and plastics, the RER will go down," says Ajay Kochhar, CEO, Li-Cycle.
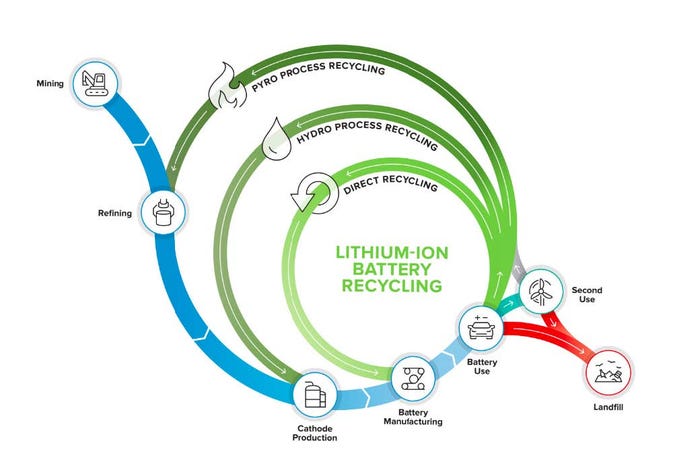
Figure 1. ReCell Center is focused on closed-loop recycling by directly recovering materials from spent batteries for
manufacturing in a process that minimizes energy use and waste. ReCell Center’s main goal is to improve the
economics via direct recycling [7].
Hydrometallurgical methods, also known as chemical leaching and extraction, are less capital- and energy-intensive and can recover lithium, but rely on large volumes of potentially environmentally harmful chemicals. Various companies, such as Accurec and Toxco Inc. (now Retriev Technologies Inc.), have developed leaching and extraction processes using these technologies. Spent Li-ion batteries are recycled through the following steps: pretreatment, leaching (typically using sulfuric acid), solvent extraction (typically using large amounts of sodium hydroxide for neutralization and additional acid for stripping), and precipitation. Multiple solvent extraction steps are necessary to separate all raw materials, and the process flow must be optimized to obtain high recovery rates (>90%) for each material. For example, Neometals first shreds batteries to obtain plastic, steel casings, and metal foil.
Next, they implement hydrometallurgy to leach and extract in the following order: copper sulfate, aluminum and iron oxide, manganese sulfate, nickel sulfate, and finally cobalt sulfate. It is possible to collect byproducts for additional sources of revenue. For instance, Neometals collects their ammonium sulfate ‘tailing,’ concentrates the material, and sells it as a liquid fertilizer. After each of the desired products and byproducts are recovered, there is generally a large amount of wastewater remaining (>10 times the amount of feedstock initially fed into the process on a mass basis). Costly processes are then needed to treat and dispose of this water and the constituent contaminants. As a result, reuse and recovery of solvents can dramatically impact the economics of the process (Neometals has an 85% solvent recovery rate). Nevertheless, these processes still require large economies of scale to have compelling unit economics (on the order of 20,000-60,000 tons).
Direct recycling is still under development, but typically relies on physical separation of battery components (such as crushing the cell) and then recovering materials based on density. Automation of sorting, disassembly, and recovery would increase efficiency. The value from directly recycled Li-ion batteries could be significantly higher by relithiating cathodes rather than fully dissolving or smelting cathodes and recovering the individual elements to then remanufacture the cathode structures. ReCell Center is pioneering the technology. Direct recycling has high potential as a cost-effective route to recover lithium iron phosphate (LFP) batteries, which offer almost no economic value because they are composed of relatively low-cost base materials. These batteries are widespread today in hand-held tools and EVs in China. LFP batteries are expected to increase in EVs in North America and Europe in the coming years for lower-cost models. The challenge of direct recycling is the fast turnover cycles of next-generation material and the high rate of change predicted for cathode technologies. It will be difficult to forecast changes in chemistry over the next 8-10 years, and consumers are likely not willing to buy EVs with dated battery chemistries and performance. Additionally, while re-lithiation of cathodes has been shown to be successful with defective or lightly-aged cathode materials, it is unclear if these regeneration processes will be able to sufficiently repair cathode structures that have undergone severe degradation over a full vehicle lifetime. Nth Cycle employs a unique electro-extraction flow-through process that uses carbon filters and electricity to recover the metals of interest. This process is inherently more energy-efficient than pyrometallurgy and hydrometallurgy and requires less volume of material to achieve profitability.
This translates to higher margins and lower price sensitivity in the short term given their lower upfront CAPEX requirements and operating costs. In particular, Nth Cycle’s modular approach can be placed onsite at existing recycling locations (separating copper, cobalt, nickel, manganese, and graphite from black mass) to improve economics and lower emissions for the final hydrometallurgy stages, or at mines to upgrade ore before transportation. Their output material (high grade hydroxides) are sold to late stage refineries to be converted into sulfates for cathode manufacturing.
References
[1] Sylvia, T. (2020, July 15). Battery adoption skyrocketed in the 2010s and lithium-ion reigns
supreme. pv magazine USA.
https://pv-magazine-usa.com/2020/07/15/battery-adoption-skyrocketed-in-the-2010s-and-lithium-ionreigns-
supreme/.
[2] Statista. (2021, February 5). Global lithium-ion battery market 2020–2025.
https://www.statista.com/statistics/1011187/projected-global-lithium-ion-battery-market-size/
[3] Circular Energy Storage Research & Consulting. (2020, December). The lithium-ion battery life
cycle report 2021. https://circularenergystorage.com/reports
[4] Statista. (2020, July 3). Lithium-ion batteries - statistics & facts.
https://www.statista.com/topics/2049/lithium-ion-battery-industry/
[5] Anderson, M. (2013b, March 1). Potential Hazards at Both Ends of the Lithium-Ion Life Cycle.
IEEE Spectrum.
https://spectrum.ieee.org/green-tech/fuel-cells/potential-hazards-at-both-ends-of-the-lithiumion-life-c
ycle
[6] Union of Concerned Scientists, & Nealer, R. (2015, November). Cleaner Cars from Cradle to
Grave: How Electric Cars Beat Gasoline Cars on Lifetime Global Warming Emissions.
https://www.ucsusa.org/sites/default/files/attach/2015/11/Cleaner-Cars-from-Cradle-to-Grave-full-rep
ort.pdf
[7] Kuntz, T. (2019, February 15). DOE launches its first lithium-ion battery recycling R&D center:
ReCell | Argonne National Laboratory. Argonne National Laboratory.
https://www.anl.gov/article/doe-launches-its-first-lithiumion-battery-recycling-rd-center-recell
[8] Oberhaus, D. (2020, December 3). The Race To Crack Battery Recycling—Before It’s Too Late.
Wired. https://www.wired.com/story/the-race-to-crack-battery-recycling-before-its-too-late/
[9] Former Tesla CTO JB Straubel tackles battery recycling with Redwood Materials. (2021, April
10). CNBC.
About the Authors
You May Also Like






.png?width=300&auto=webp&quality=80&disable=upscale)